Mini-Review Article
Tanycyte in Physiology and Disease
Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Fudan University, Shanghai, China
*Corresponding author: Song Qin, Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China, E-mail: sqin@fudan.edu.cn
Received: April 3, 2019 Accepted: April 15, 2019 Published: April 23, 2019
Citation: Hu J, Gong P, Qin S. Tanycyte in Physiology and Disease. Int J Transl Med. 2019; 1(1): 3-7. doi: 10.18689/ijtm-1000102
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Tanycytes are special ependymal cells located in the third ventricular wall and median eminence, with long processes extending into the hypothalamic parenchyma. Tanycytes have the characteristics of neural stem cells, which can self-renew or undergo neurogenesis in the adult brain. By connecting the ventricular system with blood and cerebrospinal fluid, tanycytes serve as a barrier in the brain, playing essential roles in metabolism. Tanytcytes may also be involved in reproduction and immunity. This minireview covers the recent advances of tanycytes from the aspects of morphological development and physiological functions.
Keywords: Tanycyte; Neurogenesis; Neural stem cells; Metabolism; Reproduction.
Introduction
Tanycytes are special ependymal cells located in the walls of the third ventricle (3V) and the medial eminence of the pituitary stalk funnel with long processes extending to the hypothalamus [1]. “Tanycyte” comes from “tanus” in Greek, meaning “elongated”, indicating its slender morphological characteristics. As a kind of ependymal cell, tanycytes connect the third ventricle to the pars tuberalis. In addition, they connect the cerebrospinal fluid to the pituitary portal system. Some scholars used to consider tanycytes as a kind of neuron, which have been proved wrong. Chen et al. determined that tanycytes are a specific population by single-cell RNA sequencing of mouse hypothalamus, and revealed specific markers that can distinguish them from other ependymal cells [2]. Due to the morphological and functional characteristics, tanycytes have received extensive attention in the past few decades.
Morphology and Development of Tanycytes
Tanycytesare located in the lateral walls and ventral part of 3V, with processes that penetrate the hypothalamic parenchyma, including the dorsal medial nucleus of the hypothalamus (DMH), the ventromedial nucleus (VMH), the arcuate nucleus (ARH), and median eminence (ME) [3]. According to the observation under light microscopy and electron microscopy, the ventral wall of 3V can be divided into six zones from the ventral side to the dorsal side: multi-layered arcuate tanycyte zone, monolayered arcuate tanycyte zone, irregular tanycyte zone, double layered tanycyte zone, mixed cell zone and ciliated cuboidal cell zone [4]. The apical surfaces of tanycytes have microvilli that contact cerebrospinal fluid. Rodriguez et al. described that tanycytes can be morphologically divided into α-type and β-type [5]. α-tanycytes are located in the lateral wall of 3V, mostly distributed in the dorsal part, connected with neurons and capillaries in the medial basal hypothalamic. β-tanycytes are located in the median eminence, mostly distributed in the ventral part. They have a barrier property and anatomically connect the ventricular system and their processes to the fenestrated capillaries of the median eminence. Different types of tanycytes have various properties, such as the mechanism of absorption, cargo transport, and the connection to neurons. β-tanycytes can be dominated by peptidergic and aminergic neurons, while α-tanycytes do not have such features [5]. Tanycytes specifically express the transcription factor Rax, which distinguishes it from other glial cells in the hypothalamus [2]. Tanycytes broadly express Vimentin and Nestin which also expressed by other ependymal cells in the 3V wall. So far, no protein markers have been found that can specifically label tanycytes. However, the genes Slc17a8 and Col25a1 identified by single-cell RNA sequencing may be the potential markers for the two subtypes α1 and β respectively [2]. The putative serine protease gene Prss56 is also variably expressed in tanycytes, inversely correlating expression with proopio melanocortin (POMC) in the hypothalamus [6]. In addition, SOX2, brain lipoprotein (BLBP), glutamate/aspartate transporter (GLAST), Mushashi-1, glial fibrillary acidic protein (GFAP), Notch1, Notch2, LHX2, RAX and HES5 are also expressed in tanycytes [7] (Figure 1).
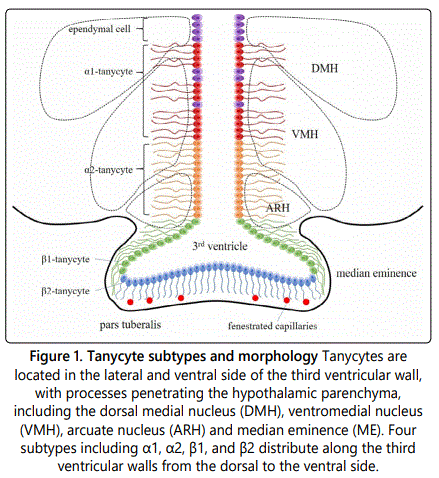
Radial glia cells function as neural progenitors during CNS development [8]. Derived from radial glia cells, tanycytes have the characteristics of neural stem cells and have been considered to be adult hypothalamic neural progenitor cells. They are proliferative in vivo with or without growth factors. They express the neural progenitor markers, such as vimentin and nestin. Tanycytes are differentiated from a subpopulation of radial glial cells in the brain of rodents [5]. Robbins et al. reported α-tanycytes in the brain of adult mice have neural stem cell activity and the location of α-tanycytes is an important neurogenic niche in postnatal hypothalamus [9]. The daughter cells can self-renew to generate more α2-tanycytes, and may also produce β-tanycytes or parenchymal astrocytes. Fibroblast growth factor (FGF) signaling pathway plays an important role in this process [9]. The β2-tanycytes located in the median eminence can produce new neurons that affect body weight and metabolism [10]. However, the division of tanycytes in the third ventricular wall of adult mice is very limited [11]. The normal differentiation and structure of tanycytes and the maintenance of the barrier between the cerebrospinal fluid and the hypothalamus require the regulation of the retinal and pro-neural pleated homeobox transcription factor (Rax). LIM homeodomain gene can maintain the Rax-dependent activation of tanycyte-specific genes, while inhibits the expression of ependymal cell-specific gene. Thus, this regulation pathway is an intrinsic key factor affecting tanycytes differentiation [12,13]. The neurogenic function of tanycytes greatly depends on the gap junction between cells. Tanycytes highly express connexin-43 (Cx43). The panglial coupling network is affected and the proliferation of hypothalamic cells is reduced in the Cx43 conditional knockout mice [14].
In the process of aging, the insulin-like growth factor (IGF)-1 signaling pathway controls tanycytes stem cell activity by regulating their cleavage pattern. The numbers of neurons are increased in the brain of IGF-1 receptor knockout mice, and the self-renewal ability of α-tanycytes increased, maintaining their stem cell activity [15]. Tanycytes also undergo morphological changes. In the early childhood to adolescence, tanycytes in the human brain connect the ventricular wall with the median eminence, arranged in an arcuate parallel with few crosses. In elderly people, tanycytes gradually lose their organization and become shorter with more crosses than infants and children [16].
Physiological Functions of Tanycytes
The distribution pattern of tanycytes allows for specific features. For example, β-tanycytes, which are distributed in the median eminence with processes extending outward and contacting the blood-brain barrier, can connect the ventricular system to the fenestrated capillaries. Thus, tanycytes can act as sensors to the concentration levels of chemicals such asglucose and hormones in the blood. Similarly, α-tanycytes on the lateral 3V walls can monitor various chemicals in the cerebrospinal fluid. The elongated shape of tanycytes also makes it close to the energy sensing neurons. Thus, in addition to acting as a neural progenitor in neurogenesis, tanycytes also play a role in the energy metabolism of organisms. Recent studies have shown that tanycytes also participate in reproduction.
Substance exchange
Langlet et al. showed that tanycytes are present in all circum ventricular organs (CVOs) and participate in the exchange of substances between blood, brain and cerebrospinal fluid [17]. Tanycytes are able to connect the ventricle to the fenestrated capillaries near the median eminence due to their elongated structural features. This connection is a diffusion barrier. Different from the blood-brain barrier, it is formed by tight junction proteins and has vascular permeability, which allows molecules in the blood to diffuse and maintain the osmotic pressure balance between both sides [18]. The barrier functions of tanycytes would be impaired if the cortex was damaged. This is because the tight junction proteins Cldn-1 and ZO-1 in tanycytes were affected with a disorganized distribution, and the osmotic pressure balance between cerebrospinal fluid and interstitial fluidwas broke [19]. In addition, Rax has an important role in maintaining the cerebrospinal fluid-hypothalamus barrier [12]. Inward-rectifier potassium channel Kir4.1 is abundantly expressed in the process of tanycytes. It transports K+ between neurons and blood vessels, and the ventricle. It may also regulate cell proliferation and differentiation from the perspective of cell migration [20].
Energy metabolism
Tanycytes play an important role in the hormonal secretion of the thyroid gland, which has an impact on the bodyʼs energy intake and consumption, especially the seasonal changes in mammalian body weight. Type II thyroxinedeiodinase (D2) is exclusively expressed by tanycytes to produce triiodothyronine (T3), indicating that tanycytes are the main source of triiodothyronine in the brain [5]. Mohácsik et al. have shown that increasing D2-mediated T3 production during E18 to P2 provides the initial required localized T3-dependent negative feedback for the developing chicken hypothalamus. T3 concentration [21]. In the case of inflammation, the NFκB signaling pathway is essential for the up-regulation of D2 in tanycytes [22]. Lewis and Ebling studies suggest that the secretion of the pineal gland is regulated by photoperiod. Melatonin changes periodically during the year, to which the pars tuberalis are highly sensitive. The pars tuberalis expresses abundant melatonin receptors and can regulate the function of tanycytes in adjacent hypothalamus by paracrine, thereby affecting the transportation and metabolism of the thyroid hormones and retinoic acids. Thus, tanycytes are an important factor in the seasonal cyclical changes in body weight [23,24]. Tanycytes can shield the pituitary by increasing the end plate size through the thyroxine receptor and G protein-coupled receptor pathways, and induce TRH to degrade extracellular enzyme activity, thereby regulating the concentration of thyroxine in the hypothalamic-pituitary-thyroid (HPT) axis from the hypothalamic level [25]. When the light time is short, cell proliferation and neurogenesis of the hypothalamus is increased. The mechanism is important for seasonal changes, and may be based on i) the neurogenic potential of tanycytes, ii) the fact that they are the locus of striking seasonal morphological changes and iii) the similarities to mechanisms involved in de novo neurogenesis of energy balance neurons. The cyclical reduction of retinoic acid and thyroxine pathway activity causes neurodegenerative and apoptosis, resulting in appetite and weight loss [26].
The expression of glucose transporter-2, K+ATP channel in tanycytes indicates that tanycytes may have the function of detecting glucose concentration in cerebrospinal fluid [5]. Glucokinase (GK) and its regulatory protein (GKRP) are present in the nucleus of tanycytes. GK is regulated in a short-term manner during nuclear differentiation, and GK can act as a molecular switch to capture cellular responses to increase glucose [27]. When the glucose concentration is lowered, or stimulated by feeding-driven neurotransmitters like histamine, acetylcholine, etc., tanycytes can respond by consuming ATP to change the intracellular Ca2+ concentration or by causing Ca2+ to oscillate. Tanycytes also respond to glucose analogs and may have an integrated effect on energy status-related signals in the body [28]. Tanycytes can also respond to other sweet substances such as acesulfame, and the sweet receptors Tas1r2 and Tas1r3 play a role in this process [29]. Due to the important role of tanycytes in glucose metabolism and neurogenesis, some scholars believe that it links Alzheimerʼs disease (AD) and type II diabetes. Type II diabetes patients often have symptoms of cognition impairment, and changes in insulin pathway activity also affect AD. Although the role of tanycytes in this is still unclear, related researches will provide new ideas for the treatment of AD and type II diabetes [7].
Tanycytes can regulate energy by affecting the permeability, and can regulate the energy homeostasis in the body through neurogenesis. In postnatal and adult mouse hypothalamus, tanycytes expressing FGF-10 are capable of generating new neurons at the appetite energy regulation center [30].
The hypothalamus plays an important role in the fat metabolism of the brain, and tanycytes can act as adjustable gates. When leaner mice received a normal diet, the lipid droplets would enter the tanycytes to synthesis neutral lipid and be stored. Some of the unsaturated fatty acids will enter the astrocytes for ketone body formation. Tanycytes show basal levels of lipid droplets (LDs) storing basal levels of neutral lipid. When obese mice received a high-fat diet, there was a significant increase in the amount of neutral lipid synthesis and lipid droplets in tanycytes. In addition, because saturated fatty acids and unsaturated fatty acids enter the astrocytes differently, the amount of them is also different. Tanycytes can play a gating role and regulate the hypothalamus for the intake of different fatty acids [31].
Tanycytes are able to respond to essential and nonessential amino acids such as arginine and lysine through the Tas1r1 and Tas1r3 umami receptors. Amino acids trigger the changes of Ca2+concentration through both receptors. They activate the P2 receptor by releasing enough ATP to continue to amplify the Ca2+ signal. Since amino acids are an important satiety signal in the body, tanycytes may reduce food intake through this pathway [32].
The leptin receptor (LePR) is present in tanycytes in the median eminence. These tanycytes are capable of transporting leptin via the ERK signaling pathway. The bodyʼs tolerance to leptin is related to obesity. This transport pathway may play a key role in the production of leptin tolerance and is of significance for the treatment of obesity [33].
Reproduction
Tanycytes are involved in the release of gonadotropinreleasing hormone (GnRH) into the portal vein, which involves the expression of estrogen receptors, the absorption of chemical molecules in the cerebrospinal fluid, and the transmission of signals to gonadotropin-releasing hormone neurons. Removal of tanycytes prevents the pulsatile release of the gonadotropin-releasing hormone, the peak of luteinizing hormone and the ovulation process [5].
Immunology
Hasegawa-Ishii et al. showed that tanycytes can express eosinophilic chemokine (Eotaxin, CCL11) and granulocyte colony-stimulating factor (G-CSF), and changes in the cytokine profile in the diencephalon may accelerate the kinetics of immune cells migrating from the bone marrow to the diencephalon. This association may have important implications for the migration immune cell and the immunity in the brain [34].
Conclusion
Tanycyte, a special type of ependymal cells which were differentiated from radial glial cells, was officially identified as a cell type in recent studies. Tanycytes are divided into two subtypes, α (including α1 and α2) and β (including β1 and β2). They have different distribution pattern and various functions. Tanycytes have the characteristics of neural stem cells and neurogenesis from early birth to adult. Tanycytes play an important role in material metabolism, including hormone secretion of the thyroid gland, detection and regulation of glucose and amino acid concentrations, and regulation of lipid metabolism. The development of tanycytes can affect the physiological functions of hypothalamus such as appetite, energy and metabolism. It is also related to the occurrence of metabolic related diseases such as obesity. The progress of study on tanycytes provides new idea for clinic treatment of metabolic related diseases. In addition, there is a link between aging-related diseases and dysfunction of tanycytes. In terms of reproduction and immunization, tanycytes also bear certain functions, but the specific mechanisms and effects are still needed for exploring in future.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No.31871477) and Natural Science Foundation of Shanghai (18ZR1403800).
References
- Prevot V, Dehouck B, Sharif A, Ciofi P, Giacobini P, Clasadonte J. The Versatile Tanycyte: A Hypothalamic Integrator of Reproduction and Energy Metabolism. Endocr Rev. 2018; 39(3): 333-368. doi: 10.1210/er.2017-00235
- Chen R, Wu X, Jiang L, Zhang Y. Single-Cell RNA-Seq Reveals Hypothalamic Cell Diversity. Cell Rep. 2017; 18(13): 3227-3241. doi: 10.1016/j.celrep.2017.03.004
- Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013; 36(2): 91-100. doi: 10.1016/j.tins.2012.12.008
- Mathew TC. Regional analysis of the ependyma of the third ventricle of rat by light and electron microscopy. Anat Histol Embryol. 2008; 37(1): 9-18. doi: 10.1111/j.1439-0264.2007.00786.x
- Rodríguez EM, Blázquez JL, Pastor FE, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005; 247:89-164. doi: 10.1016/S0074-7696(05)47003-5
- Wittmann G, Lechan RM. Prss56 expression in the rodent hypothalamus: Inverse correlation with pro-opiomelanocortin suggests oscillatory gene expression in adult rat tanycytes. J Comp Neurol. 2018; 526(15): 2444-2461. doi: 10.1002/cne.24504
- Raikwar SP, Bhagavan SM, Ramaswamy SB, et al. Are Tanycytes the Missing Link Between Type 2 Diabetes and Alzheimerʼs Disease? Mol Neurobiol. 2019; 56(2): 833-843. doi: 10.1007/s12035-018-1123-8
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004; 41(6): 881-890.
- Robins SC, Stewart I, McNay DE, et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGFresponsive neural progenitors. Nat Commun. 2013; 4: 2049. doi: 10.1038/ncomms3049
- Lee DA, Bedont JL, Pak T, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012; 15(5): 700-702. doi: 10.1038/nn.3079
- Hendrickson ML, Zutshi I, Wield A, Kalil RE. Nestin expression and in vivo proliferative potential of tanycytes and ependymal cells lining the walls of the third ventricle in the adult rat brain. Eur J Neurosci. 2018; 47(4): 284-293. doi: 10.1111/ejn.13834
- Miranda-Angulo AL, Byerly MS, Mesa J, Wang H, Blackshaw S. Rax regulates hypothalamic tanycyte differentiation and barrier function in mice. J Comp Neurol. 2014; 522(4): 876-899. doi: 10.1002/cne.23451
- Salvatierra J, Lee DA, Zibetti C, et al. The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. J Neurosci. 2014; 34(50): 16809-16820. doi: 10.1523/JNEUROSCI.1711-14.2014
- Recabal A, Elizondo-Vega R, Philippot C, et al. Connexin-43 Gap Junctions Are Responsible for the Hypothalamic Tanycyte-Coupled Network. Front Cell Neurosci. 2018; 12: 406. doi: 10.3389/fncel.2018.00406
- Chaker Z, George C, Petrovska M, et al. Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Neurobiol Aging. 2016; 41: 64-72. doi: 10.1016/j.neurobiolaging.2016.02.008
- Koopman ACM, Taziaux M, Bakker J. Age-related changes in the morphology of tanycytes in the human female infundibular nucleus/median eminence. J Neuroendocrinol. 2017; 29(5). doi: 10.1111/jne.12467
- Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol. 2013; 521(15): 3389-3405. doi: 10.1002/cne.23355
- Morita S, Furube E, Mannari T, et al. Heterogeneous vascular permeability and alternative diffusion barrier in sensory circumventricular organs of adult mouse brain. Cell Tissue Res. 2016; 363(2): 497-511. doi: 10.1007/s00441-015-2207-7
- Osterstock G, El Yandouzi T, Romanò N, et al. Sustained alterations of hypothalamic tanycytes during posttraumatic hypopituitarism in male mice. Endocrinology. 2014; 155: 1887-1898. doi: 10.1210/en.2013-1336
- Fujita A, Inanobe A, Hibino H, Nielsen S, Ottersen OP, Kurachi Y. Clustering of Kir4.1 at specialized compartments of the lateral membrane in ependymal cells of rat brain. Cell Tissue Res. 2015; 359(2): 627-634. doi: 10.1007/s00441-014-2030-6
- Mohácsik P, Füzesi T, Doleschall M, et al. Increased Thyroid Hormone Activation Accompanies the Formation of Thyroid Hormone-Dependent Negative Feedback in Developing Chicken Hypothalamus. Endocrinology. 2016; 157(3): 1211-1221. doi: 10.1210/en.2015-1496
- de Vries EM, Kwakkel J, Eggels L, et al. NFκB signaling is essential for the lipopolysaccharide-induced increase of type 2 deiodinase in tanycytes. Endocrinology. 2014; 155(5): 2000-2008. doi: 10.1210/en.2013-2018
- Lewis JE, Ebling FJ. Tanycytes as Regulators of Seasonal Cycles in Neuroendocrine Function. Front Neurol. 2017; 8: 79. doi: 10.3389/fneur.2017.00079
- Ebling FJ. Hypothalamic control of seasonal changes in food intake and body weight. Front Neuroendocrinol. 2015; 37: 97-107. doi: 10.1016/j.yfrne.2014.10.003
- Müller-Fielitz H, Stahr M, Bernau M, et al. Tanycytes control the hormonal output of the hypothalamic-pituitary-thyroid axis. Nat Commun. 2017; 8(1): 484. doi: 10.1038/s41467-017-00604-6
- Helfer G, Barrett P, Morgan PJ. A unifying hypothesis for control of body weight and reproduction in seasonally breeding mammals. J Neuroendocrinol. 2019; 31(3): e12680. doi: 10.1111/jne.12680
- Salgado M, Tarifeño-Saldivia E, Ordenes P et al. Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes. PLoS One. 2014; 9(4): e94035. doi: 10.1371/journal.pone.0094035
- Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol. 2011; 589: 2275-2286. doi: 10.1113/jphysiol.2010.202051
- Benford H, Bolborea M, Pollatzek E, et al. A sweet taste receptordependent mechanism of glucosensing in hypothalamic tanycytes. Glia. 2017; 65(5): 773-789. doi: 10.1002/glia.23125
- Haan N, Goodman T, Najdi-Samiei A, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013; 33(14): 6170-6180. doi: 10.1523/JNEUROSCI.2437-12.2013
- Hofmann K, Lamberz C, Piotrowitz K, et al. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia. 2017; 65(2): 231-249. doi: 10.1002/glia.23088
- Lazutkaite G, Soldà A, Lossow K, Meyerhof W, Dale N. Amino acid sensing in hypothalamic tanycytes via umami taste receptors. Mol Metab. 2017; 6(11): 1480-1492. doi: 10.1016/j.molmet.2017.08.015
- Balland E, Dam J, Langlet F, et al. Hypothalamic tanycytes are an ERKgated conduit for leptin into the brain. Cell Metab. 2014; 19(2): 293-301. doi: 10.1016/j.cmet.2013.12.015
- Hasegawa-Ishii S, Inaba M, Li M, et al. Increased recruitment of bone marrow-derived cells into the brain associated with altered brain cytokine profile in senescence-accelerated mice. Brain Struct Funct. 2016; 221(3): 1513-1531. doi: 10.1007/s00429-014-0987-2


